On June 2, 2025, Escugen presented the latest data from the Phase I clinical trial of ESG401 (Trop2 ADC) in advanced solid tumors at the 2025 ASCO meeting in the form of a poster.
Title:Comprehensive Phase 1 Results of ESG401, a TROP2-Targeting ADC, in Advanced Solid Tumors.
Background:ESG401 is a novel ADC comprising a humanized anti-TROP2 IgG1 monoclonal antibody conjugated to SN-38, with a Drug-Antibody Ratio of 8 and a stable cleavable linker. The antitumor efficacy and good tolerability of ESG401 demonstrated in the Phase 1a trial and in patients with brain metastases have been published in the internationally renowned medical journals Cell Reports Medicine and ESMO Open, respectively. This report summarizes the latest results of the Phase I clinical trial of ESG401 in advanced solid tumors.
Methods:
This study is an open-label, dose-escalation, and dose expansion Phase I trial. Eligible patients are males/females aged 18 to 75 years, with an ECOG performance status of 0-1, and have been histologically or cytologically confirmed to have advanced or metastatic solid tumors. Patients receive intravenous infusion of ESG401 according to the specified dosing regimen (every 21 or 28 days as one cycle) and continue treatment until disease progression, intolerable toxicity occurs, the subject withdraws consent, the subject dies, or other reasons specified in the protocol.
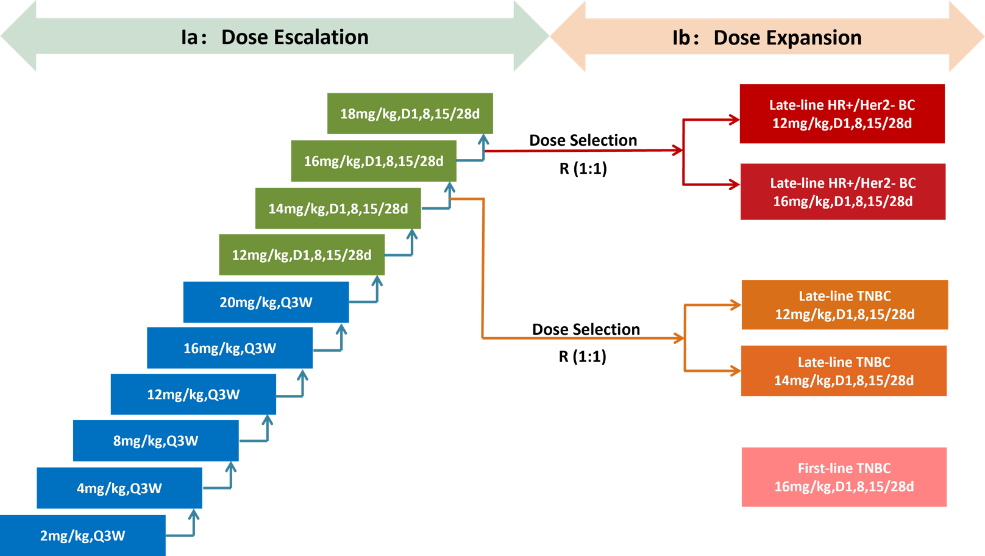
Results:
Baseline Characteristics
As of Oct 23, 2024, 156 pts received ≥1 dose of ESG401, including 40 patients in Phase 1a and 116 patients in Phase 1b. The median age of the enrolled patients was 52.5 years (range: 32–73). Sixty-eight percent of the patients had an ECOG performance status (PS) of 1. Sixty-seven percent of the patients had received two or more prior lines of therapy in metastatic setting, and 18% had received five or more prior lines of therapy. At baseline, 57%, 54%, and 13% had liver, lung, and brain metastases, respectively.
Safety
The safety profile was consistent with previous reports, showing no new or unexpected safety signals. The most common treatment-related adverse events (TRAEs) of Grade ≥3 included leukopenia and neutropenia.
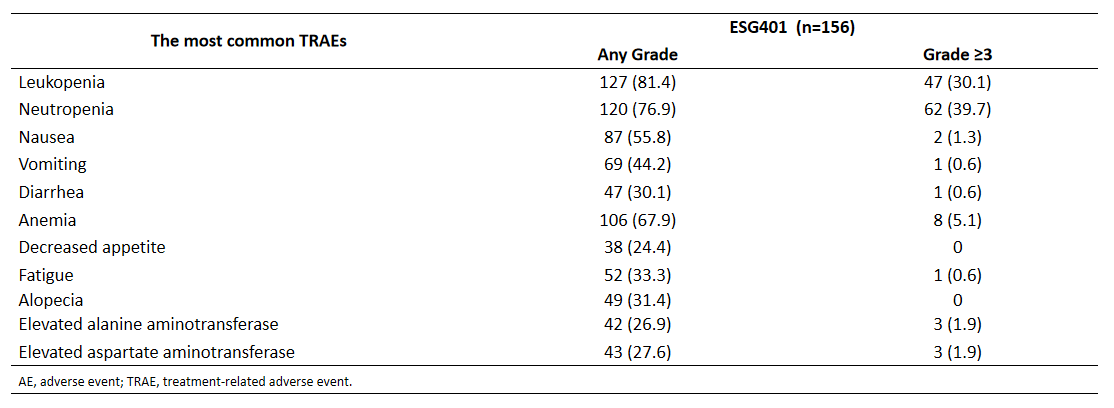
Efficacy
In the cohort of patients with metastatic triple-negative breast cancer (mTNBC) receiving ESG401 as first-line treatment, 35 evaluable subjects achieved an objective response rate (ORR) of 83%, a disease control rate (DCR) of 100%, and the median progression-free survival (PFS) has not yet been reached. Among patients with HR+/HER2- breast cancer receiving ESG401 as later-line treatment, 58 evaluable subjects achieved an ORR of 34.5%, a DCR of 77.6%, and a median PFS of 7.4 months. In patients with advanced triple-negative breast cancer, the ORR was 35.1%, the DCR was 62.2%, and the median PFS was 3.7 months. Additionally, in patients with HER2+ breast cancer, endometrial cancer, and adenoid cystic carcinoma receiving ESG401 as later-line treatment, the DCR was 100% in each indication.
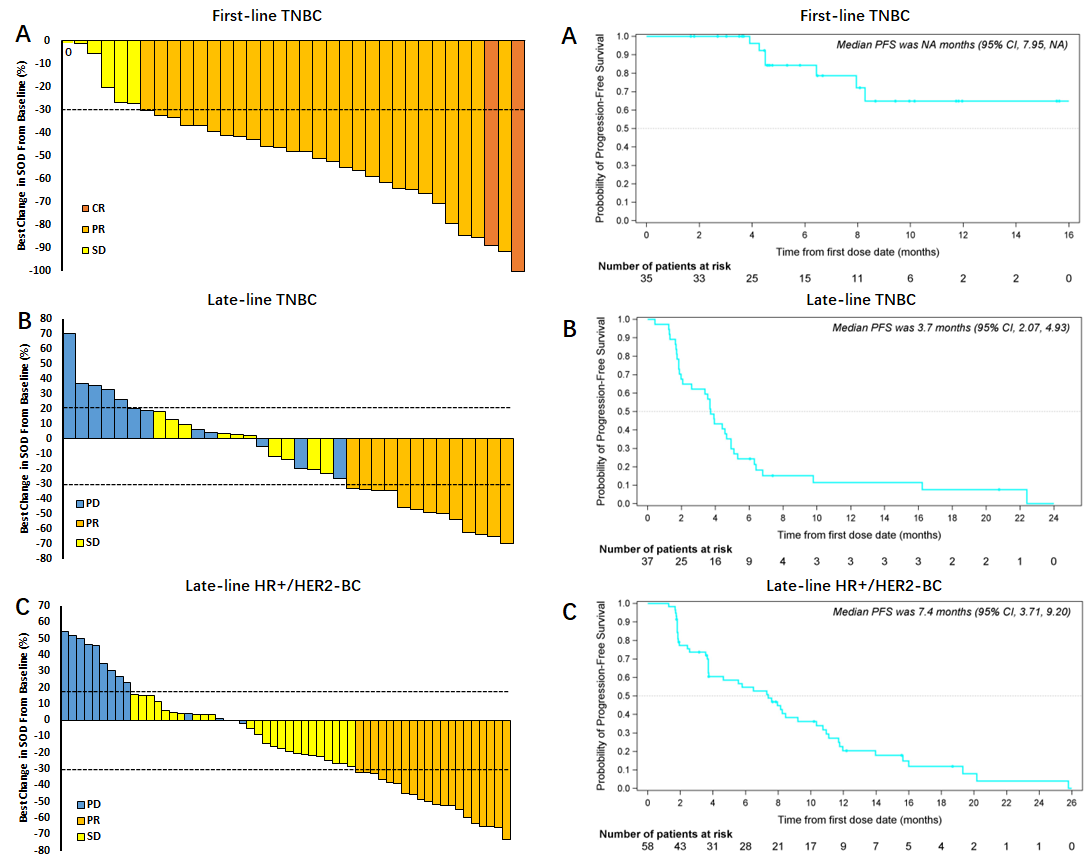
Figure 1. Antitumor Activity of ESG401
L: Waterfall plot of best percent change in SOD in target lesions in patients with (A) First-line TNBC, (B) Late-line TNBC and (C) Late-line HR+/HER2–BC. R: Kaplan-Meier plots of progression-free survival in patients with (A) First-line TNBC, (B) Late-line TNBC and (C) Late-line HR+/HER2–BC.
Highlights Summary:
ESG401 is the first clinical-stage ADC from Escugen, featuring an innovative, stable, and cleavable linker that significantly reduces off-target toxicity. Clinical data show that the main adverse reactions of ESG401 are imperceptible and easily manageable decreases in white blood cells and neutrophils. The incidence of Grade 3 or higher anemia is low. Grade 3 or higher diarrhea and oral mucositis are only rarely reported. No cases of interstitial pneumonia have been observed. The tolerable dose of ESG401 is much higher than that of other ADCs targeting the same antigen, with low incidence and mild severity of both off-target and on-target toxicities, demonstrating a significant safety advantage. The increased ADC treatment dose and in-body exposure have led to encouraging efficacy in patients with various types of breast cancer, as well as endometrial cancer and adenoid cystic carcinoma. These results warrant further clinical investigation.
The original ESG401 poster text from the ASCO meeting:
