On June 2, 2025, Escugen's ESG401 (Trop2 ADC) was first reported at the 2025 ASCO meeting in the form of a poster, presenting the clinical trial data of ESG401 in salivary gland cancer.
Title:Anti-TROP2 ADC ESG401 in a Master Protocol Clinical Trial for Salivary Gland Cancer Based on Molecular Typing.
Background:
Salivary gland carcinomas (SGC) are rare, with limited prospective data and no standard or FDA-approved systemic therapy for recurrent/metastatic disease; gene-targeted precision therapy is emerging as a promising strategy. This report presents the preliminary results of ESG401 as a TROP2-targeted therapy for the treatment of salivary gland carcinoma (SGC).
Methods:
This study is a single-center, open-label master protocol trial (NCT06145308) to evaluate molecular subtype-guided precision neoadjuvant, transformative, or rescue therapy for SGC. Participants who are TROP2-positive will receive treatment with ESG401.
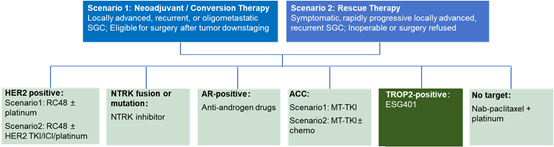
SGC, Salivary Gland Carcinoma; HER2, Human Epidermal Growth Factor Receptor 2; TKI, Tyrosine Kinase Inhibitor; ICI, immune checkpoint inhibitor; AR, Androgen Receptor; ACC, Adenoid Cystic Carcinoma; MT, Multitarget.
Results:
Enrollment and Baseline Characteristics
As of the cutoff date (Jan 22, 2025), 14 pts in the cohort received ≥1 dose of ESG401. The median age of the enrolled patients was 48.5 years (range: 31–65); there were 7 males and 7 females (50% each). At baseline, 43%, 7%, and 29% had lung, liver, and brain metastases, respectively. Seventy-nine percent of the patients had previously undergone surgery, 43% had received radiotherapy, 36% had received chemotherapy, and 14% had not received any prior treatment.
Safety:
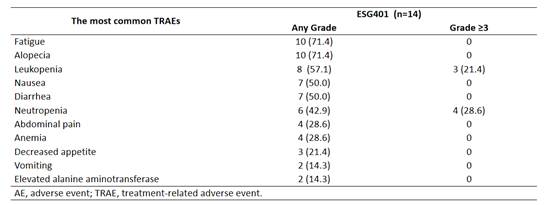
Fatigue and alopecia were the most reported adverse events in patients with salivary gland carcinoma. The only grade ≥3 adverse events observed were leukopenia and neutropenia.
Efficacy:
In the cohort of patients receiving ESG401 as neoadjuvant/transformational therapy, 4 subjects were evaluable for efficacy, with a disease control rate (DCR) of 100%. Among patients with salivary gland carcinoma receiving ESG401 as rescue therapy, 8 subjects were evaluable for efficacy, achieving an objective response rate (ORR) of 50% and a DCR of 100%.In 2 evaluable patients with brain metastases at baseline, the intracranial ORR reached 100%, with 1 patient achieving a complete response (CR).
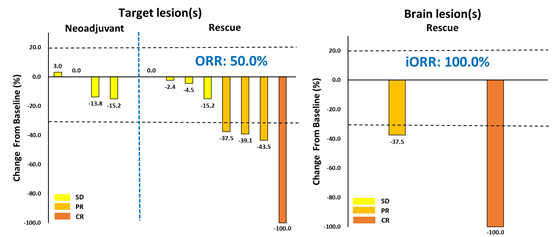
Figure 1. Waterfall plot of best percentage change in the sum of diameters (SOD) of target and brain lesions.
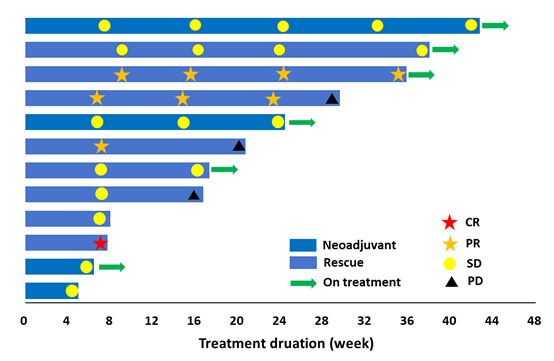
Figure 2. Swimmer plot of all efficacy-evaluable subjects.
Highlights Summary:
In the field of salivary gland carcinoma (SGC), where treatment options are extremely limited, ESG401 as a TROP2-targeted therapy has demonstrated significant clinical potential. Clinical data show that for patients with advanced SGC, including those with poor-prognosis brain metastases, ESG401 has achieved breakthrough antitumor activity, resulting in encouraging responses (including complete remission) and excellent disease control. Additionally, positive disease control outcomes were observed in the neoadjuvant/transformational therapy setting, suggesting its potential for use in earlier treatment stages. In terms of safety, ESG401 exhibited a manageable safety profile, with the most common adverse events being fatigue and alopecia (both Grade 1-2). The only Grade ≥3 adverse reactions observed were hematologic toxicities (leukopenia and neutropenia). Overall, ESG401 has shown excellent antitumor effects and controllable safety in patients with advanced SGC (including brain metastases), offering new hope for this refractory patient population. These results support the rationale for targeted therapy based on molecular subtypes in this patient group and warrant further validation through larger-scale clinical studies.
The original ESG401 poster text from the ASCO meeting:
