
On October 20, 2025, Escugen presented the latest clinical data of ESG401 (a Trop2 ADC) as first-line treatment for metastatic triple-negative breast cancer in a poster at the ESMO 2025 Congress. As early as November 2024, ESG401 had already received Breakthrough Therapy Designation for this indication from the Center for Drug Evaluation (CDE) of China's National Medical Products Administration.
Study Title: Updated Efficacy of Anti-TROP2 ADC ESG401 for First-Line Metastatic TNBC.
Background
ESG401 is a Trop-2 ADC composed of a humanized Trop-2 antibody conjugated with SN38 (DAR=8) with a stable-cleavable linker. ESG401 showed efficacy in heavily pretreated metastatic breast cancer (mBC). However, data remain limited for its specific use in the first-line (1L) metastatic triple-negative breast cancer (mTNBC), particularly as monotherapy. We hereby update results from the 1L mTNBC cohort of a Phase 1a/1b study (NCT04892342).
Method
Patients (pts) aged ≥18 years with confirmed local advanced/unresectable or metastatic TNBC and no prior treatment in the metastatic setting received ESG401 (16 mg/kg IV on D1, 8, 15/28 days) until progression, unacceptable toxicity, or withdrawal of consent.
Results
Patient Enrollment and Baseline Characteristics
As of June 30, 2025, 40 patients had received at least one dose of ESG401. The median age of enrolled patients was 54 years (range: 33-73). 20% of patients were newly diagnosed with stage IV disease. At baseline, 58% of patients had lung metastases, 38% had liver metastases, and 8% had brain metastases. Additionally, 25% of patients had received neoadjuvant chemotherapy, 78% had undergone surgery, 68% had received adjuvant chemotherapy, and 38% had received radiotherapy.
Safety
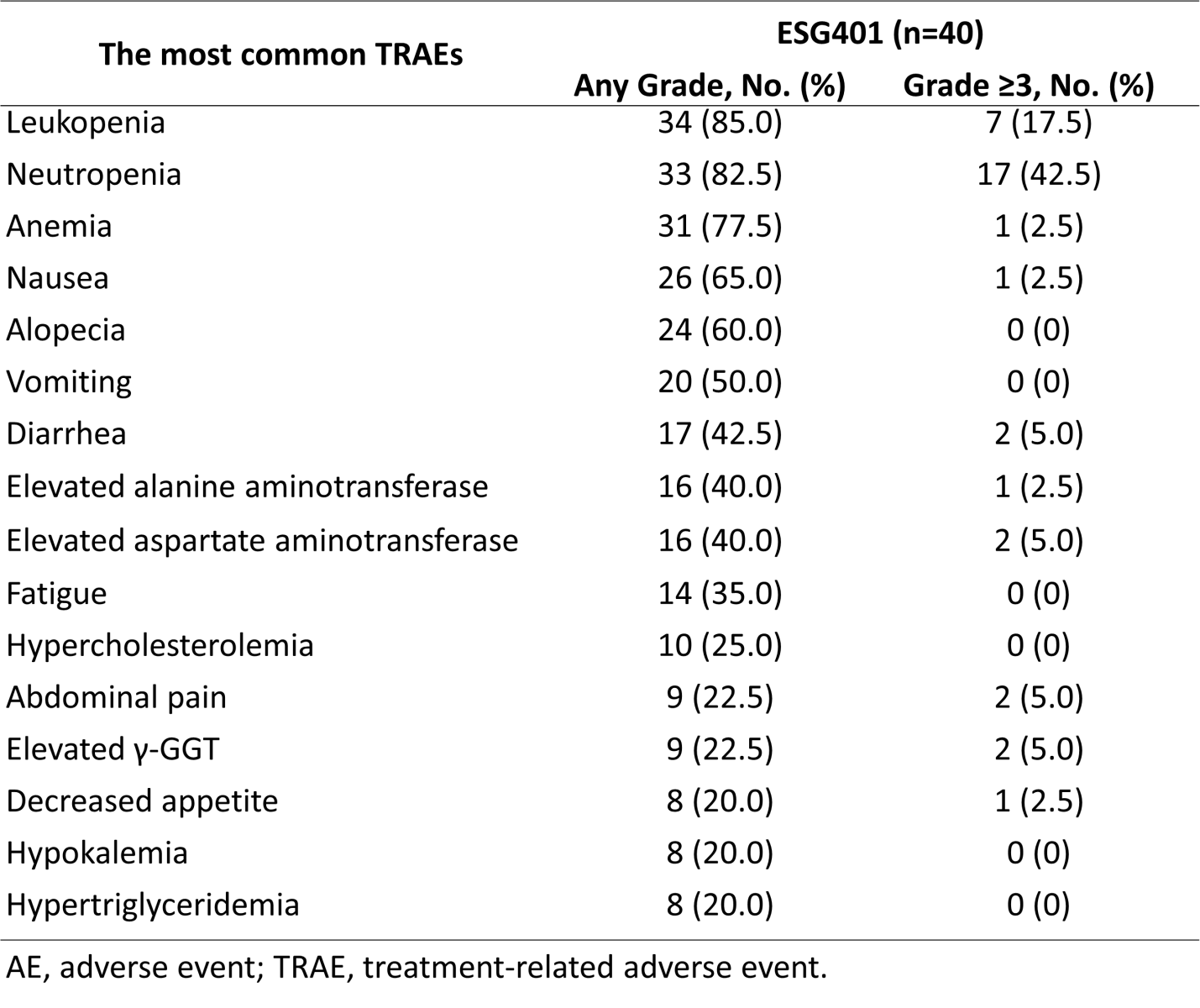
The safety profile was consistent with previous reports, showing no new or unexpected safety signals. The most common grade ≥3 treatment-related adverse events (TRAEs) included leukopenia and neutropenia.
Efficacy
Of the 40 mTNBC patients receiving ESG401 as first-line treatment, 35 were evaluable for efficacy. Among these, 3 subjects achieved a complete response (CR). The objective response rate (ORR) was 82.9%, disease control rate (DCR) was 100%, with median progression-free survival (PFS) of 13.0 months and median duration of response (DOR) of 18.8 months.
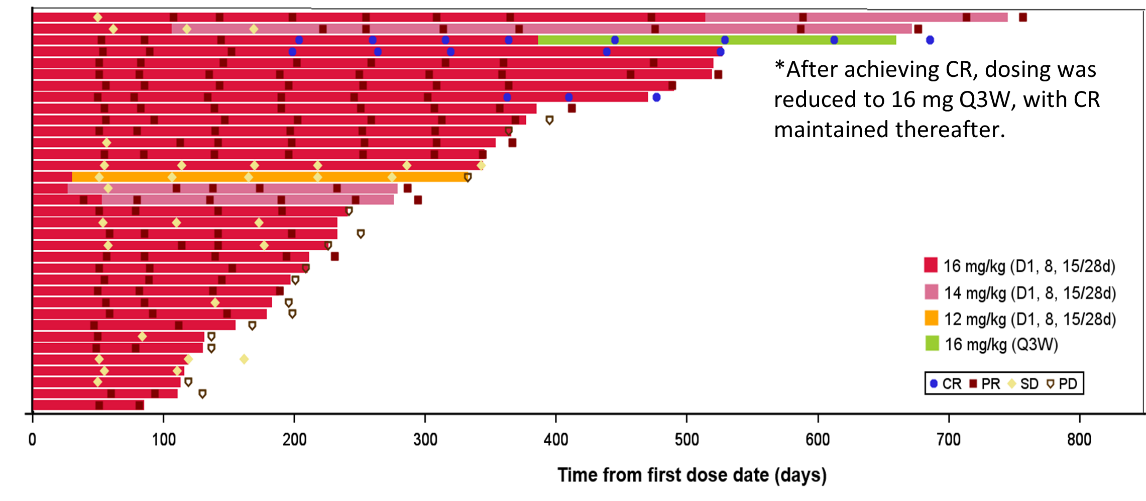
Fig 1. Swimmer plot of individual patient.
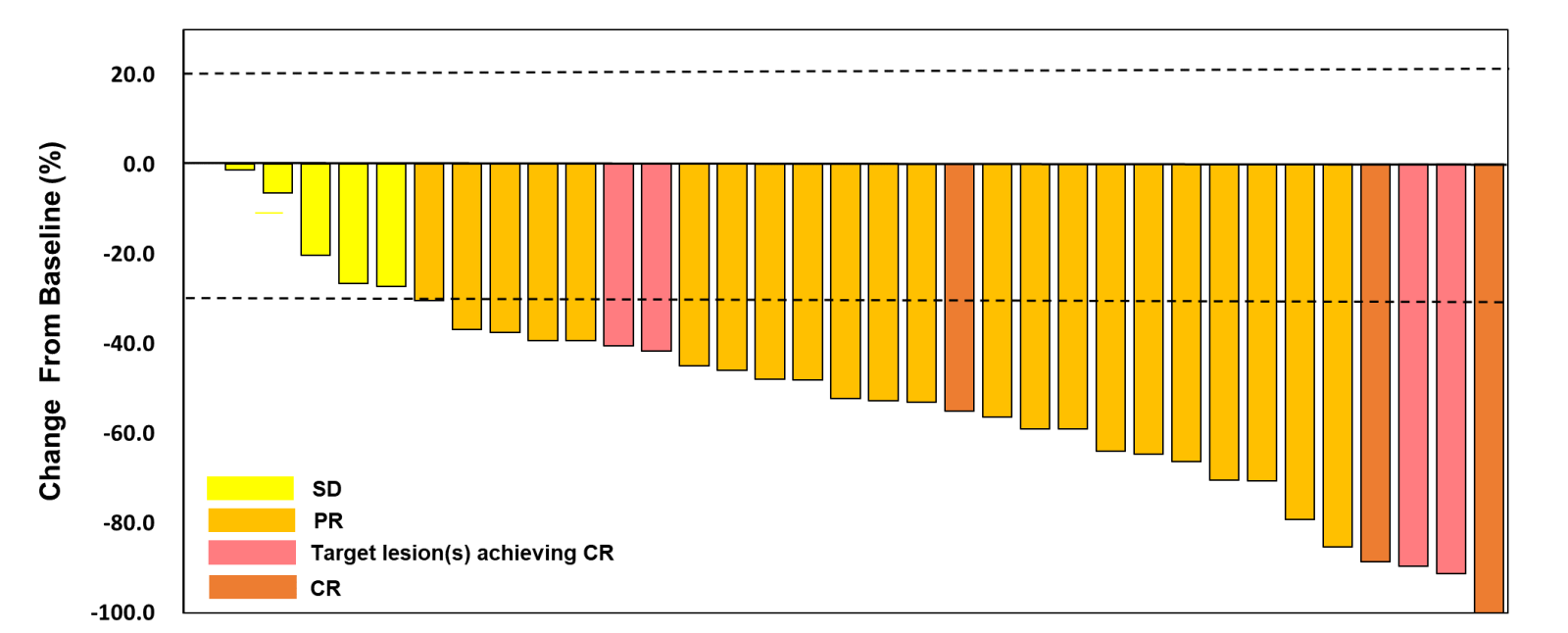
Fig 2. Waterfall Plot of Best Percentage Change in Sum of Target Lesion Diameters (SOD).
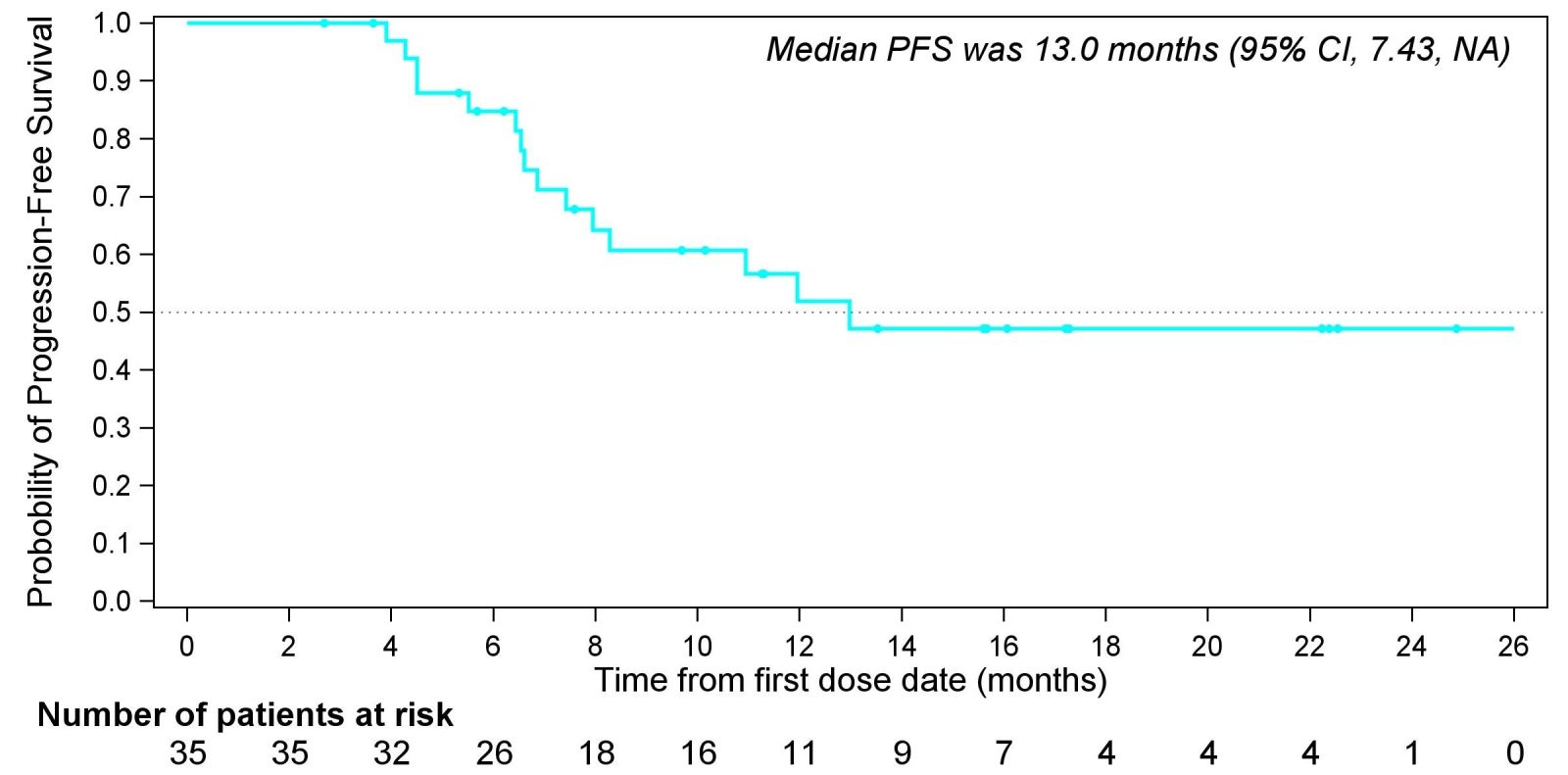
Fig 3. Kaplan-Meier plots of progression-free survival.
Key Highlights
At this ESMO conference, Gilead and AstraZeneca/Daiichi Sankyo also presented the Phase III clinical trial results of Sacituzumab Govitecan (ASCENT-03 study) and Dato-DXd (TROPION-Breast02 study) for the first-line treatment of triple-negative breast cancer (TNBC), respectively. The author has summarized the findings as follows:

The original poster of ESG401 presented at the ESMO Congress is as follows:
