From May 30 to June 3, 2025, the largest and most prestigious global oncology conference—the American Society of Clinical Oncology (ASCO) Annual Meeting—will be held at the McCormick Place Convention Center in Chicago, USA. Escugen’s TROP2 ADC product ESG401 will present two research findings at this year’s conference. This marks the third consecutive year that the clinical progress of ESG401 has been selected for presentation at the ASCO Annual Meeting.
Comprehensive Phase 1 Results of ESG401, a TROP2-Targeting ADC, in Advanced Solid Tumors
Background: ESG401 is a novel ADC comprising a humanized anti-TROP2 IgG1 monoclonal antibody conjugated to the Topoisomerase I inhibitor SN-38 via a stable cleavable linker. ESG401-101 is a phase 1, open-label, dose-escalation (1a) and dose-expansion(1b) study evaluating the safety and antitumor activity of ESG401 in advanced solid tumors. This report summarizes the comprehensive phase 1 results.
Methods: Patients (pts) aged 18–75 years with locally advanced/metastatic solid tumors received ESG401 until unacceptable toxicity, progressive disease, or consent withdrawal. Phase 1a results (n=40) have been reported previously. Phase Ib comprised three parallel cohorts: late-stage TNBC, late-stage HR+/HER2-, and first-line TNBC.
Results: As of Oct 23, 2024, 156 pts were enrolled at 13 sites across China (40 in 1a; 116 in 1b). Most pts had metastatic HR+/HER2-BC (n=65; median prior lines: 3; range: 1–10), followed by late-line TNBC (n=47; median prior lines: 3; range: 1–12), first-line TNBC (n=40), HER2+BC (n=2), and one case each of endometrial cancer (EC) and adenoid cystic carcinoma (ACC). All pts had distant metastases at baseline; 13%, 57%, and 54% had brain, liver, and lung metastases, respectively. ESG401 demonstrated efficacy (Table) in pts with solid tumor, including those with brain metastases. The safety profile remained consistent with no new or unexpected signals. The most common any-grade TEAEs were leukopenia, neutropenia, anemia, nausea, and vomiting. Grade ≥3 TRAEs were primarily neutropenia and leukopenia, none leading to permanent discontinuation. TRAEs led to delayed dosing, dose reduction, and discontinuation in 38.5%, 7.1%, and 2.6% of pts, respectively.
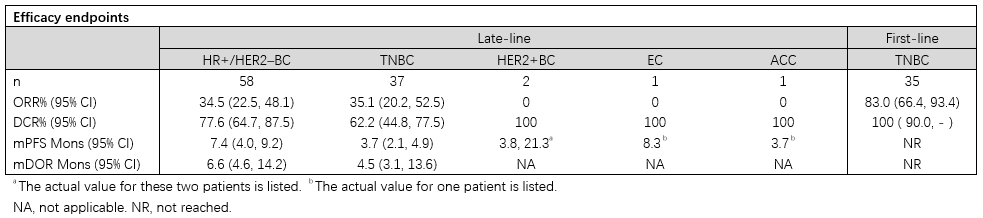
Conclusions: ESG401 demonstrated favorable safety and efficacy benefits due to its enhanced linker, showing good safety and promising antitumor activity in advanced solid tumors across settings. These results warrant further clinical investigation. Clinical trial information: NCT04892342.
Anti-TROP2 ADC ESG401 in a Master Protocol Clinical Trial for Salivary Gland Cancer Based on Molecular Typing
Background: Salivary gland carcinomas (SGC) are rare, with limited prospective clinical outcome data. There is no standard of care or FDA-approved systemic therapy for recurrent and/or metastatic (R/M) disease. Precision therapy targeting specific gene alterations is emerging as a promising approach for SGC. We designed a single-center, open-label, master protocol clinical trial evaluating the efficacy and safety of molecular subtype-guided precision neoadjuvant/transformative or rescue therapy for SGC (NCT06145308). This report focuses on the preliminary results of the TROP2-targeted group.
Methods: Pts with locally advanced/recurrent SGC received neoadjuvant/transformative therapy, while pts with locally advanced/recurrent who could not tolerate or refused surgery/radiotherapy and pts with symptomatic, rapidly progressive metastatic SGC received rescue therapy. Pts were divided into molecular subtypes (HER2, NTRK, AR, TROP2) or assigned to chemotherapy if no molecular alterations were detected. Trop2-positive pts were assigned to the TROP2 group and treated with ESG401 (16 mg/kg i.v. on days 1, 8, and 15 of each 28-day cycle). The primary endpoint was ORR per RECIST1.1; secondary endpoints included AEs, DCR, PFS, and OS.
Results: As of Jan 22, 2025, 14 Trop2-positive pts were enrolled, including 4 receiving neoadjuvant/transformative therapy and 10 receiving rescue therapy. Among the 12 efficacy-evaluable pts, pathological types included salivary duct carcinoma (n=3), adenoid cystic carcinoma (n=5), and others (n=4). Safety findings were consistent with the ESG401-101 study, with no new safety signals observed. Among 4 pts receiving neoadjuvant/transformative therapy, 2 achieved decreased SD, though not meeting PR criteria. Among 8 pts receiving rescue therapy, 4 achieved PR with ORR was 50% (4/8). For 12 efficacy-evaluable pts, DCR was 100% (12/12). Three pts with brain metastases achieved IC-PR/CR, yielding an IC-ORR of 100%.
Conclusions: ESG401 demonstrated promising efficacy in Trop2-positive SGC, providing a rationale for molecular subtype-based targeted therapy in this population and warranting further investigation in larger studies.
Clinical trial information: NCT06145308.