On May 13, 2025, the clinical research results of ESG401, a TROP2 ADC drug developed by Escugen Biotechnology for treating brain metastasis in patients with advanced HER2-negative breast cancer, were officially published online in ESMO Open, a well-known international medical journal hosted by the European Society for Medical Oncology (ESMO). The paper is titled "Novel TROP2 antibody–drug conjugates for treatment of HER2-negative metastatic breast cancer patients with brain metastases: a promising option." The article reports the efficacy and safety data of ESG401 in HER2-negative breast cancer patients with brain metastasis.
Original link:
https://www.sciencedirect.com/science/article/pii/S2059702925009287
Patients and methods:This subgroup analysis was conducted as part of an open-label, multi-dose, dose-escalation, and cohort-expansion multicenter phase I trial. Eligible participants were aged 18-75 years and had locally advanced or metastatic solid tumors. For this subgroup analysis, patients with histologically confirmed HER2-negative BC and BMs were enrolled. Efficacy endpoints included overall objective response rate (ORR), disease control rate (DCR), and progression-free survival (PFS). Intracranial-specific endpoints included intracranial ORR (iORR), intracranial DCR (iDCR), and intracranial PFS. This trial is registered at ClinicalTrials.gov, NCT04892342.
Results:Among 17 patients with efficacy-evaluable BMs, the iORR was 41% (7/17) [95% confidence interval (CI) 18.4% to 67.1%] including 3 patients achieving an intracranial complete response. The iDCR was 76% (13/17) (95% CI 50.1% to 93.2%). The overall ORR was 53% (9/17) (95% CI 27.8% to 77.0%), the overall DCR was 71% (12/17) (95% CI 44.0% to 89.7%), and the medium PFS was 5.7 months. The safety profile was consistent with previous reports.
Conclusions:These findings suggest that ESG401 is a promising and well-tolerated treatment option for BMs.
Introduction:
Brain metastases (BMs) occur in 20%-40% of patients with metastatic breast cancer (mBC), ranking second after lung cancer. The relatively high incidence of BMs in mBC patients may be attributed to improved survival owing to better control of extracranial disease using other treatments, such as targeted therapy and advanced imaging techniques. Patients with metastatic triple-negative (TNBC) or human epidermal growth factor receptor 2 (HER2/EGFR2)-overexpressing BC develop BMs more frequently than patients with hormone receptor (HR)-positive, i.e. luminal subtype, BC. BMs are a significant risk factor for mortality and are associated with a poorer prognosis and reduced quality of life compared with those for patients without BMs. Initial treatment for BMs usually consists of local therapy, including surgical resection, post-operative radiotherapy, stereotactic radiosurgery (SRS), and/or whole-brain radiotherapy (WBRT).However, the distinct microenvironment of BMs, with varied cell types and metabolic constraints, as well as limited drug penetration through the blood–brain barrier (BBB), often results in poor control of BMs with most systemic therapies. Though there is a lack of specifically designed and adequately powered prospective interventional trials to support their use in these patients with BMs, antibody–drug conjugates (ADCs) demonstrate clinically relevant intracranial activity in BMs in a variety of trials, particularly exemplified by trastuzumab deruxtecan in HER2-positive BC. To date, there are established systemic treatment recommendations for BMs in HER2-positive BC, such as tucatinib and trastuzumab deruxtecan. However, there is no standard systemic treatment for BMs in patients with HER2-negative BC; hence, clinical trials of mBC commonly exclude patients with progressive BMs because of concerns about poor efficacy, shortened life expectancy, or increased risk of toxicity. This highlights the critical unmet clinical need for innovative treatments for BMs in patients who represent a poor prognosis cohort.
ESG401 is an innovative and optimally designed ADC drug composed of a humanized anti-trophoblast cell-surface antigen 2 (TROP2) immunoglobulin G1 monoclonal antibody conjugated to a topoisomerase I inhibitor SN-38 via a proprietary novel stable linker and achieves an average drug–antibody ratio of 8. The optimized design of ESG401 is expected to minimize toxicity and improve the therapeutic index. Additionally, ESG401 is scheduled to have minimized ‘on-target off-tumor’ and ‘off-tumor’ toxicity using a stable linker and moderate toxic payload, thus effectively reducing toxic side-effects. Consequently, ESG401 is expected to exhibit an optimal therapeutic index and accurately address the challenges associated with current TROP2 ADC products.
Despite the presence of an intact BBB, some systemic therapies have limited ability to cross it. However, in the case of BMs, changes caused by the metastatic process may enhance vascular permeability, allowing the extravasation of large molecules such as ADCs across the BBB. Preclinical studies of ESG401 indicated that it can reduce the size of BMs in mouse models, suggesting that ESG401 may have therapeutic potential against BMs. Consequently, patients with BMs were eligible for enrollment in the phase I study of ESG401 (NCT04892342), which aims to explore the safety and efficacy of ESG401 in patients with BMs at an early stage of clinical development.
Here, we describe a subgroup analysis and present the safety and efficacy findings for patients with stable BMs in the phase I study. In this trial, patients with stable BMs showed a positive intracranial response to ESG401. These findings prompted us to further investigate the potential of ESG401 and TROP2 ADCs in treating BCBMs. The complete results of the phase I study are scheduled to be reported separately.
Patients and methods
Study design
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines and local institutional review board at the participating site. The study was approved by the ethics committees of all sites. The ethics committee approval number of the leading site, the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, is 21/264-2935. Written informed consent was provided by all patients before enrollment.
The study was designed as an open-label, multi-dose, dose-escalation, and cohort-expansion multicenter phase I/II trial (NCT04892342). It consisted of three parts: dose-escalation (phase Ia), cohort expansion (phase Ib), and primary efficacy exploration (phase II). The patients enrolled in a dose-escalation study with planned 10 dose levels were administered intravenously at predetermined doses of 2-20 mg/kg once every 3 weeks, or 12-18 mg/kg on days 1, 8, and 15 in a 4-week cycle until disease progression, death, or unacceptable toxicity. Dose escalation continued until the identification of the maximum tolerated dose (MTD) or the predicted efficacy dose if an MTD is not identified, owing to the paucity of dose-limiting toxicities. The phase Ib study is designed to include six cohorts, with each planned to enroll 20-50 subjects who have specific tumor types, including TNBC and HR-positive/HER2-negative BC. Patients with stable or new BMs were eligible to be enrolled. ESG401 was administered intravenously until unacceptable toxicity, progressive disease (PD), or withdrawal of consent. Both extracranial and intracranial disease status were assessed using RECIST 1.1 by investigator assessment. Subjects included in this subgroup analysis were patients with BMs at baseline who were included in this study.
Human subjects
Patients with histologically confirmed HER2-negative BC with BMs were enrolled in this subgroup analysis. Demographic information is provided in Supplementary Table S1.
Patient eligibility
Eligible patients were male and non-pregnant, non-lactating females aged 18-75 years who had been diagnosed with locally advanced or metastatic solid tumors. Patients without symptomatic central nervous system (CNS) metastases or those not requiring ongoing treatment for CNS metastases, including steroids and antiepileptic agents, were enrolled. Other key criteria included adequate hematological, liver, and renal function, and no known history of anaphylactic reactions to irinotecan or gastrointestinal diseases (such as chronic gastritis, chronic enteritis, or gastric ulcers). More detailed inclusion and exclusion criteria can be found in the study protocol in the Supplementary Material.
Endpoints
The primary endpoint of the study was to assess the safety and tolerance of ESG401 in patients with solid tumors. The subgroup efficacy endpoints included the overall objective response rate [ORR, complete response (CR) + partial response (PR)] as assessed by the investigator, disease control rate [DCR, CR + PR + stable disease (SD) based on RECIST 1.1], duration of response (DOR), depth of response (DepOR, the maximum percentage change in the sum of the longest diameters of the target lesions compared with baseline), progression-free survival (PFS), and overall survival (OS). The intracranial equivalent efficacy endpoints, intracranial ORR (iORR; CR + PR assessed according to RECIST 1.1 on intracranial lesions), intracranial DCR (iDCR; CR + PR + SD assessed according to RECIST 1.1 on intracranial lesions), intracranial DepOR (iDepOR), and intracranial PFS (iPFS) were assessed at the same time.
Safety profile analysis
Treatment-emergent adverse events (TEAEs) were categorized according to the Medical Dictionary for Regulatory Activities, version 25.1 or later, and their severity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 5.0). A safety monitoring committee was established to evaluate all safety events, timing, and relatedness to study the treatment of all patients.
Statistical analysis
Statistical analyses evaluating endpoints for all patients receiving the recommended dose have been published and are also applicable to the BM subgroup. The statistical analysis plan is provided in the Supplementary Material. The Clopper–Pearson method was used to calculate the two-sided 95% confidence interval (CI) for the ORR and DCR. The Kaplan–Meier method was used to estimate the distribution of time-to-event endpoints of DOR, PFS, and OS rate at various time points, and their corresponding two-sided 95% CIs for the median time-to-event endpoints were calculated using the Brookmeyer and Crowley methods. Statistical analysis was carried out using Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, NC) or later.
Results
Patient characteristics
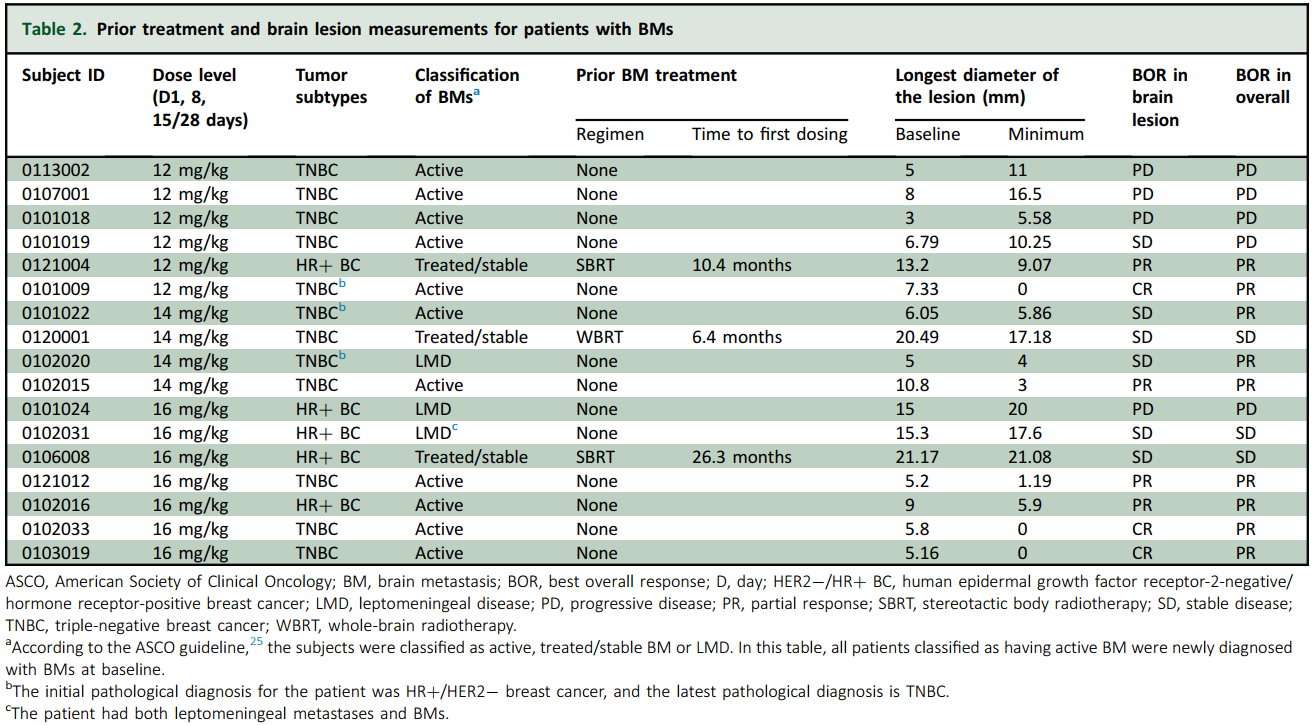
As of 17 November 2024, a total of 156 patients were enrolled in the study. Among these patients, 21 had BMs at baseline, accounting for 13.5% of all enrolled patients. In patients with BMs, the median age was 49 years, and 16 patients had at least one BM lesion with a minimum size of 5 mm. A significant proportion (>80%) of patients in the BM subgroup had an Eastern Cooperative Oncology Group performance status of 1. All patients had HER2-negative BC, with 14 patients (67%) having TNBC and 7 patients (33%) HR-positive/HER2-negative BC. The median diameter of brain lesions was 8 mm, with a maximum of 21.17 mm. Other demographic and clinical characteristics of patients in the BM subgroup are presented in Supplementary Table S1.
Before enrollment in the study, the treatment of BMs was as follows: three patients underwent radiotherapy, and the remaining patients had not received any treatment (Supplementary Table S1). Among the 17 efficacy-assessable patients, 12 had been diagnosed with the TNBC subtype as per their latest molecular pathology report, whereas the remaining 5 patients had been diagnosed as HR positive/HER2 negative. Interestingly, among these 12 TNBC patients, 3 were initially diagnosed as HR positive/HER2 negative; however, their molecular subtype later transformed into TNBC. At the time of data cut-off (17 November 2024), 3 of 17 patients with BMs (18%) were still receiving ESG401 treatment.
Efficacy
The phase Ia dose-escalation study has been completed, and the phase Ib expansion study is currently ongoing. A total of 156 patients have been enrolled in the entire phase I study, with outcomes from the 40 patients enrolled in the phase Ia study already published. In 130 HER2-negative BC patients receiving therapeutically relevant doses in phase I, the ORR, DCR, and clinical benefit rate are 50%, 88%, and 60%, respectively.
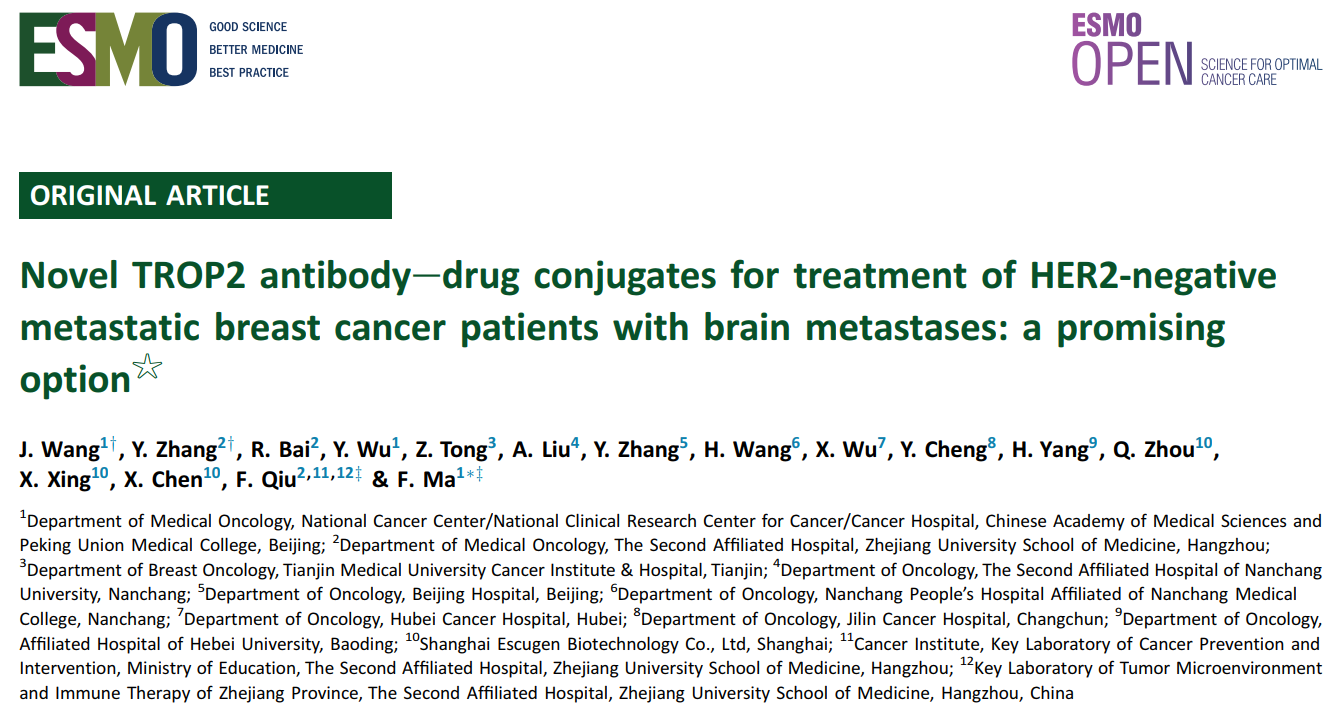
For the subgroup with BMs, all 17 patients who completed at least one post-treatment assessment were included in the efficacy analysis. The median follow-up time was 23.9 months. The ORR was 53% (9/17) (95% CI 27.8% to 77.0%), the overall DCR was 71% (12/17) (95% CI 44.0% to 89.7%), and the medium DepOR was 45.5% (range 5.0%-85.5%). The median overall PFS was 5.7 months, and the median iPFS was 9.8 months. (For patients who discontinued treatment due to an overall efficacy evaluation of PD, despite their brain lesions not meeting the PD criteria at that time point, their iPFS was censored at the date of discontinuation of treatment because no subsequent intracranial imaging assessments were carried out.) The OS was not yet available. In 11 patients with active BMs, these rates were 54.5% (6/11) for iORR and 72.7% (8/11) for iDCR. In three patients with stable/treated BMs, the iORR was 33.3% (1/3), and the iDCR was 100% (3/3). In three patients with leptomeningeal metastases, the iORR was 0% (0/3), and the iDCR was 66.7% (2/3).
Figure 1 presents the DepORs in the 17 patients with BMs. The iORR was 41% (7/17) (95% CI 18.4% to 67.1%), the iDCR was 76% (13/17) (95% CI 50.1% to 93.2%), and the medium iDepOR was 34.4% (range 0.4%-100%).
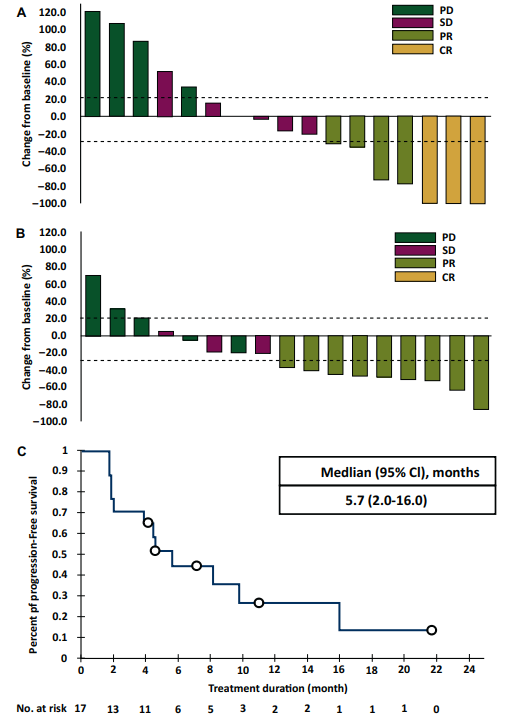
Figure 2 shows the magnetic resonance imaging scans of the brain lesions for two typical patients. In these patients, the median time to respond was 1.8 months (range 1.7-1.8 months), and the median iPFS was 8.0 months (95% CI 4.7-16.0 months).
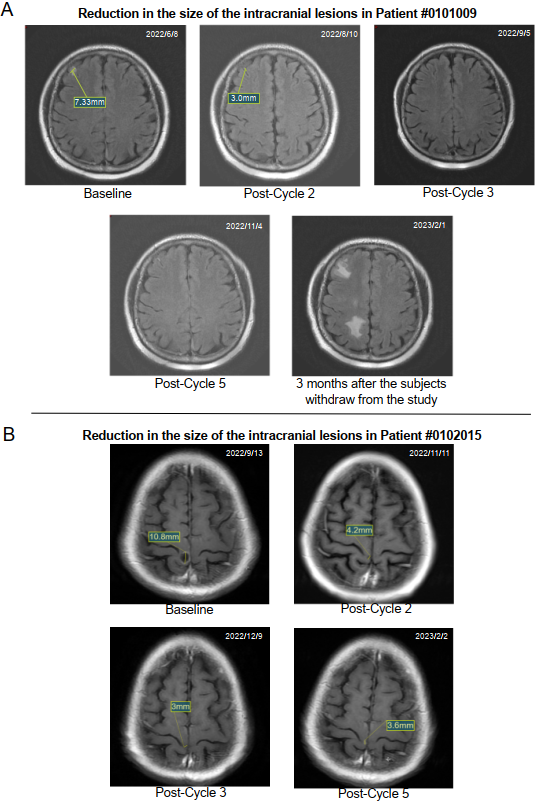
Detailed information on the summary of treatment efficacy at various dosages, prior treatment, and brain lesion measurements is listed in Tables 1 and 2.
Among the 17 patients in the BM subgroup, 9 demonstrated an overall efficacy of PR. Of these nine patients, the evaluation of brain lesions showed that seven had a PR or CR and two had a reduced SD. This indicates that the nine BM patients who responded to ESG401 treatment also exhibited a consistent response in their brain lesions, demonstrating that the response to ESG401 treatment is aligned in both systemic and intracranial settings. Furthermore, a comparison of efficacy measurements among patients with BMs, those without BMs, and the overall patient population in the phase I study showed that the efficacy in the subgroup with BMs is not inferior to that of patients without BMs and the overall population (Supplementary Tables S2 and S3).
Safety profile
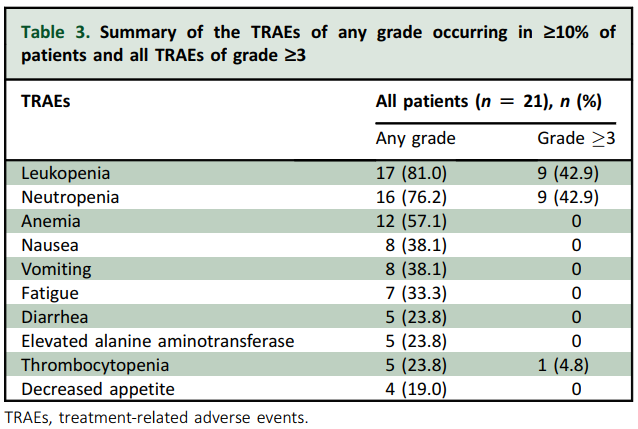
All 21 patients experienced at least one TEAE. The treatment-related adverse events (TRAEs) of any grade were predominantly hematological or gastrointestinal, with leukopenia (81.0%), neutropenia (76.2%), anemia (57.1%), nausea (38.1%), vomiting (38.1%), fatigue (33.3%), diarrhea (23.8%), and thrombocytopenia (23.8%) being the most common adverse events. Grade ≥3 TRAEs were reported in 42.9% of patients, including neutropenia (42.9%) and leukopenia (42.9%) (see Table 3). No grade ≥3 thrombocytopenia, diarrhea, skin rash, or oral mucositis was observed, and no case of interstitial lung disease was reported. No TRAE associated with discontinuation or death occurred. The safety profile is consistent with that in the previously published report. Overall, ESG401 was well tolerated in the BM subgroup, and its safety profile was consistent with that in the previously published reports of patients without BMs.
Discussion
BMs are a devastating consequence occurring in 20%-40% of patients with mBCs, and their occurrence can vary significantly across different BC subtypes. The average OS of patients with BC after the diagnosis of BMs ranges from 2 to 16 months, with ∼80% patients dying within 1 year. For the control of HER2-positive BC patients with BMs, it is recommended that patients with diffuse lesions or extensive metastases and those with single lesions exhibiting significant clinical symptoms first consider local treatments, including surgery, stereotactic graded radiotherapy, WBRT, and SRS. On this basis, systemic treatment with targeted drugs has been administered.
Classical chemotherapy agents, such as capecitabine, cyclophosphamide, 5-fluorouracil, methotrexate, vincristine, cisplatin, etoposide, vinorelbine, and gemcitabine, continue to play a significant role in the management of BMs from BC. However, their efficacy remains suboptimal, and there are still considerable unmet clinical needs. In recent years, the prognosis for patients with BCBMs has improved, particularly in HER2-positive subtypes. Nevertheless, systemic treatment options for HER2-negative BCBMs have been rarely reported. Owing to the lack of effective trial data and the absence of approved systemic treatment regimens for HER2-negative BC patients with BMs, there remains a significant unmet clinical need for new targeted pharmacological treatments for this patient population.
In the development of the clinical trial for ESG401, we were particularly focused on whether ESG401 could be effective in treating BMs from the early stages of trial design. According to existing reports, ∼80% of tumors from patients with HER2-negative mBC express high levels of TROP2. Additionally, a search of the pre-The Cancer Genome Atlas (TCGA) public repository revealed that TACSTD2, which encodes TROP2, is highly expressed in the HER2-negative molecular subtype compared with the HER2-positive subtype. Furthermore, preclinical studies of ESG401 observed a reduction in BMs in mouse models. Consequently, we included patients with BMs in the phase I trial.
Our subgroup analysis of ESG401-101 provided preliminary evidence of the efficacy of ESG401 in treating BMs in heavily pretreated patients with BC, including those with TNBC and HR-positive/HER2-negative BC, highlighting its potential utility in the management of mBC. The subgroup analysis further confirmed the findings of other ADCs, such as trastuzumab deruxtecan, which demonstrated that ADC drugs can penetrate both the BBB and the blood–tumor barrier, thus providing a potential treatment option for BMs. Specifically, it was observed that despite previous treatment failure with trastuzumab deruxtecan, the patient continued to show a positive response to ESG401 for both non-CNS lesions and BM lesions. Furthermore, the results of this study indicated that the response of brain lesions in patients is consistent with the response of extracranial lesions, suggesting that brain lesions, like primary and metastatic lesions in other parts of the body, express the same molecular target, TROP2. This constitutes one of the molecular bases for the action of ESG401 as a TROP2-targeted ADC.
The data analyzed in this subgroup study revealed a notably higher ORR (53% versus 3% versus 28%) and a more significant brain lesion response rate (RR) (41% versus unknown versus 22%) in patients with BMs compared with the subgroup analysis data of the same class of drugs, sacituzumab govitecan, reported in the ASCENT trial, and datopotamab deruxtecan reported in the TROPION-Lung05 trial. The data also demonstrated a higher RR compared with previously reported treatments for this difficult-to-treat patient population, such as poly (ADP-ribose) polymerase inhibitor combination chemotherapy (intracranial RR was 12%),immune checkpoint inhibitors plus chemotherapy (overall ORR was 39.0%),and cyclin-dependent kinase 4/6 inhibitors (iORR was 5.2%).In addition to the observed treatment effects on BMs in BC patients from the phase I study, internal unpublished data from an investigator-initiated trial of ESG401 in salivary gland cancer showed promising results. Among two metastatic salivary gland cancer patients with BMs, one achieved CR of the brain lesions, and the other achieved PR, resulting in a brain ORR of 100%. This further confirms the efficacy of ESG401 in treating BMs, not only in BC patients but also in patients with other tumor types.
To the best of our knowledge, this study represents the independent detailed report on the efficacy of TROP2 ADC in the treatment of BCBMs. This study aligns with the Food and Drug Administration’s recommendation to include such patients in early clinical trials, rather than excluding them, in order to gain better insights into the potential future applications of the drug, given the high incidence of BMs in BC patients.
Our consideration of ESG401’s therapeutic effect on BMs is based on several key factors. Firstly, ADC drugs are capable of penetrating the BBB compromised by tumor metastasis, allowing them to exert therapeutic effects. This has been demonstrated in trials of trastuzumab deruxtecan for HER2-positive BC. Secondly, TROP2 expression remains relatively high in BCBMs, providing a solid foundation for TROP2-targeted ADCs like ESG401 to be effective. Lastly, and perhaps most critically, compared with other ADC drugs, ESG401 has a wider therapeutic window and a better safety profile, enabling higher therapeutic dosing. This increased dosing could lead to higher drug concentrations both in circulation and within the local BM lesions, potentially resulting in more effective treatment of BMs. Of course, these are our current hypotheses regarding the potential mechanism of ESG401 in treating BMs, and further investigation will be conducted in subsequent experiments.
This study has some limitations. Firstly, the small sample size and the fact that some patients, particularly those recruited during the dose determinate phase, may still be receiving doses below the recommended phase II dose level could have impacted the results. This is evidenced by the lower RR and DCR in patients receiving 12 mg/kg compared with those receiving 14 mg/kg and 16 mg/kg. The experience of treating lung cancer BMs with gefitinib also suggests that higher doses are necessary for patients with BMs. Secondly, the study was a retrospective subgroup analysis that did not incorporate the Response Assessment in Neuro-Oncology Brain Metastases criteria for assessing the efficacy of patients with BMs. Future prospective trials should address this limitation.
Conclusions
In conclusion, these findings from this subgroup analysis of the ESG401-101 study demonstrated the potential of ESG401 as an effective treatment option for HER2-negative BC patients with BMs. These positive findings provide a basis for further investigation of ESG401 in larger, randomized controlled trials to establish its efficacy and safety profile within this significant potential of BMs. If confirmed, ESG401 may offer a novel treatment option for BC patients with BMs, addressing a critical unmet clinical need in this population.
About Escugen Biotechnology
Escugen is a clinical-stage biotechnology company located in Shanghai, China, focusing on the development of innovative ADC (Antibody-Drug Conjugate) drugs. Currently, Escugen's most advanced Trop-2 ADC pipeline, ESG401, has entered Phase III clinical trials. Escugen's next-generation linker-payload technology platform, EZWi-Fit®, offers significant competitive advantages in terms of safety, efficacy, anti-multiple drug resistance, and pharmacokinetic characteristics. Leveraging this platform technology, Escugen is rapidly expanding its ADC product pipeline targeting new or validated targets. The company has also successfully licensed this platform technology to several domestic and international biotechnology companies to empower their innovative ADC projects.